August 16, 2024
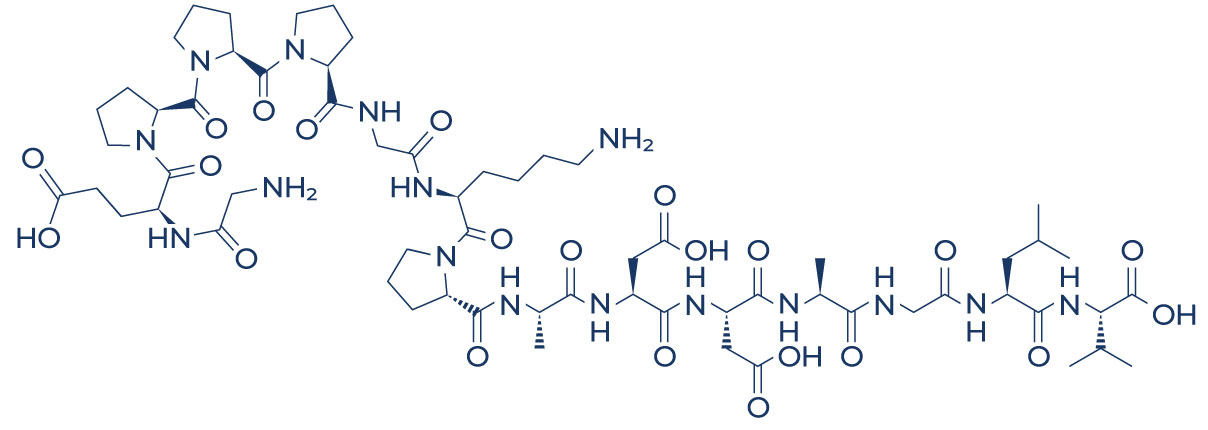
Bpc 157 And Capillary Bentham Scientific Research
Bpc 157 And Blood Vessels Bentham Science It's essential to review the pros and cons with your doctor prior to deciding on the recommended approach of management. BPC-157 can be administered by mouth, topically, or via subcutaneous injections. While dental management is convenient, injections have a tendency to give even more regular and trusted outcomes as the peptide is soaked up directly right into the bloodstream. Your doctor https://ewr1.vultrobjects.com/pharma-marketing-strategies/Pharmaceutical-quality-control/pharmacology/what-is-bpc-157-prospective-usages.html can help identify one of the most ideal management approach based upon your certain wellness goals and preferences. BPC-157 helps grow brand-new tiny blood vessels and safeguards the internal walls of capillary.What Is Bpc-157 Peptide? Is It Safe & What Is It Made Use Of For?
Stable Gastric Pentadecapeptide BPC 157 Therapy for Primary Abdominal Compartment Syndrome in Rats - Frontiers
Stable Gastric Pentadecapeptide BPC 157 Therapy for Primary Abdominal Compartment Syndrome in Rats.

Posted: Sun, 12 Dec 2021 08:00:00 GMT [source]
- BPC157 gradually deteriorated right into small molecular pieces and finally right into solitary amino acids, which got in the metabolic blood circulation in vivo.
- Peer-reviewed magazines equip compelling stories of BPC-157's restorative influence, repainting a dazzling image of its capacity.
- This consists of modulation of development aspects, cytokines, and various other molecular paths involved in inflammation and cells repair.
- This may be an early, essential factor for attaining the more complete healing result.
Musculoskeletal And Cells Healing With Bpc 157
In summary, this impact might be the reason or a repercussion of the advantageous effects of BPC 157 on associated disruptions [1,2,3,4,5,6,7,8,9,10,11] As shown, BPC 157 combats totally free radical development and complimentary radical-induced lesions [32, 82,83,84] An intriguing point would certainly be making use of the exact same dose array in BPC 157 researches [1,2,3,4,5,6,7,8,9,10,11] Lastly, further studies should make clear the molecular pathways entailed and extend the one-time application (much like the engraftment of neural stem cells [16] or bone marrow stromal cells [17] right into the lesion website) to the constant application for the recovery of pre-existing spine injury. We focused on the healing effects of the secure stomach pentadecapeptide BPC 157 in spine injury using a rat version. If you have questions concerning treatment, bills, or clinical insurance coverage, please call us by entering your information.Telehealth appointments are offered! Keep informed concerning potential therapy choices and review them openly with your healthcare provider. In spite of the FDA's ban, lots of are still interested by BPC 157's reported health and wellness benefits. While the FDA has actually banned BPC 157, it remains to be a topic of passion because of its supposed health and wellness advantages. This area dives into the positive results and potential of BPC 157, clarifying why it has been valued by many, in spite of regulatory difficulties. Significantly, BPC 157 additionally lowers the effects of, i.e., gastrointestinal and/or liver lesions (Ilic et al., 2010; Ilic et al., 2011a; Ilic et al., 2011b; Lojo et al., 2016; Drmic et al., 2017) and severe muscle weak point (Klicek et al., 2013; Medvidovic-Grubisic et al., 2017)). Hence, these helpful results are related and appear beneficial for the therapy of numerous vicious cycles that may simultaneously appear in rats permanently kept under severe intra-abdominal hypertension conditions. On their own, all these disturbances, which were ameliorated/reduced, are rather severe. Thinking about the various reasons for secondary stomach compartment syndrome (Seeker and Damani, 2004; Hedenstierna and Larsson, 2012), these disruptions, each with a different set of causes, may likewise add to high intra-abdominal stress, and therefore when ameliorated/reduced, they might suggest the useful result of BPC 157 therapy in cases of additional high intra-abdominal pressure. Based on a well-known sensation in peripheral nerve injury (i.e., as the number of preserved motoneurons lowers, the MUP (gigantic capacity) in the tail muscle increases), it is imaginable that the BPC 157-treated rats that underwent spine injury and were subjected to EMG recordings displayed a markedly lower MUP in the tail muscular tissue than that in the equivalent controls (Table 3). Consistently, the motor nerve conduction study confirmed the lack of demyelinated processes in the tail back nerves after spine injury (the CMAP revealed regular biphasic possibilities, similar amplitudes, and comparable transmission rates in all of the rats) (Table 4). While the value of this finding stays to be determined, it is probably worth stating that a reduction in the variety of huge myelinated axons in rat caudal nerves was observed in all pets till day 30, with a noticeably majority in controls and fewer in injured rats that received BPC 157 therapy. Surprisingly, after 180 days, recuperation happened, and the number of big myelinated axons in the controls reached that in the BPC 157-treated rats, and this finding persisted via completion of the experiment (Fig. 6). To additionally explore the systems through which BPC-157 might apply its improvement effects on spreading, migration, and tube formation of endothelial cells, a Signal Transduction PathwayFinder ™ RT2 Profiler ™ PCR Variety was used. There, due to its valuable impact on damaged muscle and the recuperation of its feature (Staresinic et al., 2006; Novinscak et al., 2008; Mihovil et al., 2009; Pevec et al., 2010; Kang et al., 2018), it is feasible that the BPC 157 restorative impact might likewise be related to improvements in stomach wall compliance. Both BPC 157 routines ( µg and ng) had a comparable healing result in all of the checked out methods of abdominal compartment syndrome. More cause-consequence proof might be seen in BPC 157-treated rats with high intra-abdominal stress, as treatment largely abrogated both arterial and venous apoplexy.Is BPC-157 peptide risk-free?
Upgraded: October 9, 2023. The experimental peptide BPC-157 is banned under the World Anti-Doping Agency (WADA) Prohibited Checklist in the category of S0 Unapproved Substances. Furthermore, this substance is not approved for human scientific use by any global regulatory authority and it might cause unfavorable health and wellness impacts ...
Social Links