September 5, 2024
Tesofensine, A Novel Antiobesity Medication, Silences Gabaergic Hypothalamic Neurons Pmc
Randomized Regulated Trial Of Tesomet For Weight Management In Hypothalamic Weight Problems European Journal Of Endocrinology Both these compounds have long removal half-lives (e.g., 200 h), a delayed start of optimal plasma focus (and presumed brain focus), and have receptor kinetics characterized by a sluggish countered from the receptor. The results observed with these substances epitomize many of the methodology and analysis problems seen with atypical stimulants. Subjective and unbiased actions were assessed for 48 h after each medicine management. The research study results revealed that the effects of d-amphetamine were substantially above those of placebo on all primary and secondary subjective actions. The effects of tesofensine and GSK were not substantially different from those of placebo and were less than those of d-amphetamine 30 mg on all main and most additional measures. The impacts of tesofensine were either lower than or otherwise different from those of bupropion or atomoxetine; a comparable outcome was seen with GSK compared to pseudoephedrine.Information Evaluation
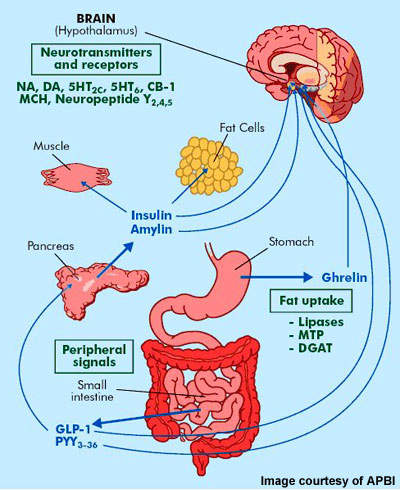
When it come to power equilibrium-- consumption versus expense-- these four phenotypes control body weight. Trick motorists for energy expenditure are relaxing power expenditure, nonexercise exercise, exercise and the thermogenic result-- increase in the metabolic price that happens after a meal-- of food and exercise. Notably, the fat burning accomplished with Tesofensine seems lasting over the long-term. Follow-up studies have actually reported upkeep of weight management even after discontinuation of therapy, recommending long-term effects on metabolic policy and hunger control. The device of action of Tesofensine as a clinical fat burning option Click here for more revolves around its inflection of neurotransmitter levels in the brain.Detailed Testimonial Of Present And Forthcoming Anti-obesity Medicines
What is the heart rate of tesofensine?
After 24 weeks, tesofensine 0.25 and 0.5 mg/day had no substantial result on systolic and diastolic blood pressures compared to placebo, however heart rate raised by 7.4/ min.
- Chow enhanced dopamine efflux in chow-fed controls, but no more in cafeteria diet-fed rats, an indicator of food reward tolerance.
- One interesting searching for in the tesofensine research was that regardless of the lack of considerable "at this moment" drug preference, topics reported dramatically better next day overall readiness to "take drug again" contrasted to sugar pill.
- One person randomized to Tesomet developed extreme paranoia and stress and anxiety after ~ 5-- 8 weeks of therapy.
- Twenty-four-week observed adjustment in research laboratory safety information in the safety and security populace of a randomized professional test of Tesomet for hypopituitary people with hypothalamic obesity.
- SAR has finished phase I trials in healthy volunteers and individuals with T2D199,200,201.

Social Links