September 5, 2024
Dual- And Triple-acting Agents For Treating Core And Co-morbid Symptoms Of Major Depression: Unique Ideas, New Medications
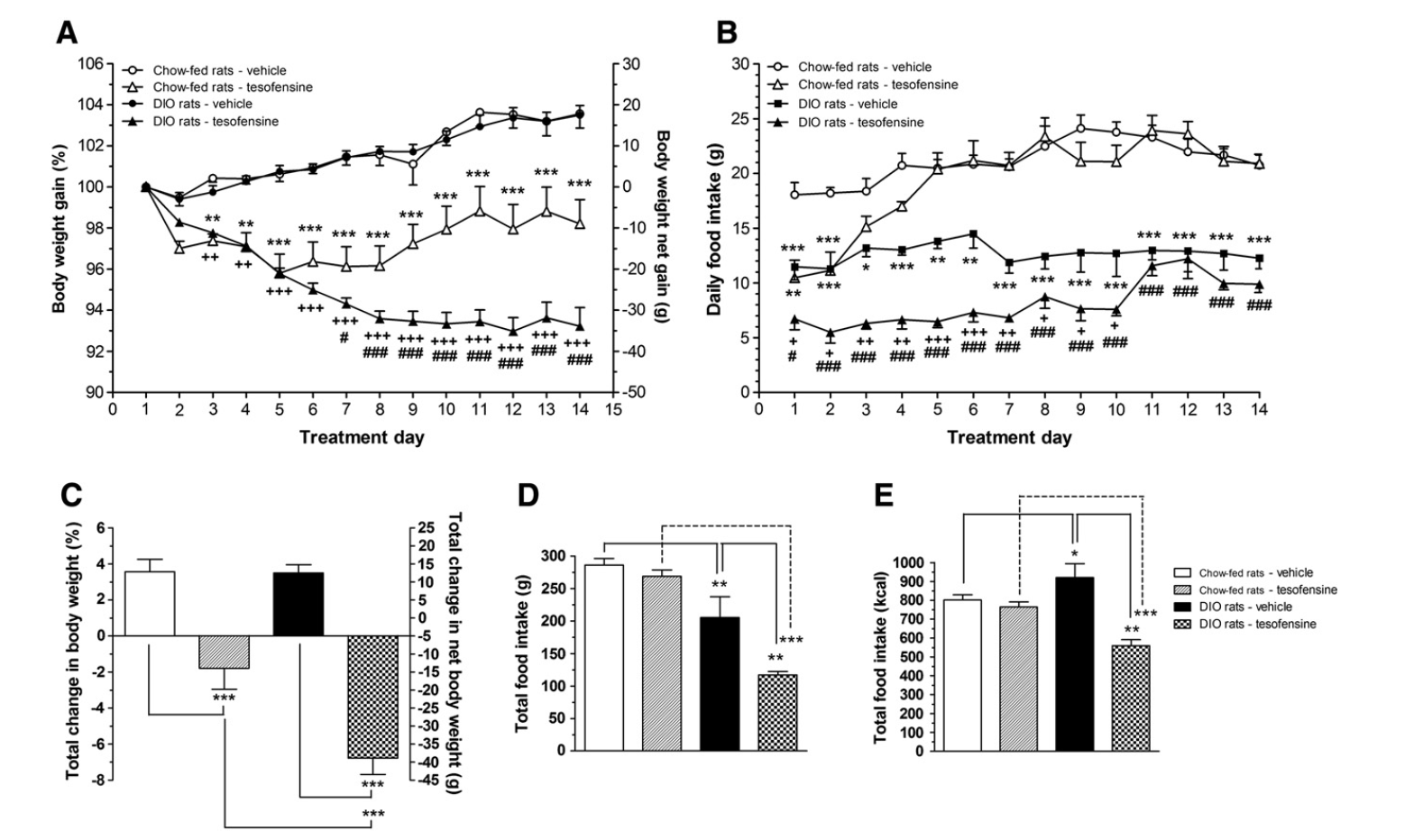
Tesofensine, An Unique Antiobesity Drug, Silences Gabaergic Hypothalamic Nerve Cells Pmc Tesofensine was reported to have a good safety and security account and was well tolerated although an increased variety of negative occasions (e.g., enhanced heart price and blood pressure) were observed in the highest possible dose teams of 0.5 mg and 1.0 mg. NeuroSearch167 specified that no medically relevant cardiovascular adverse events or adjustments in either high blood pressure or pulse were seen, according to FDA standards. Nevertheless, in researches in Parkinson's illness reduced body weight and elevated heart rate were described as usual in Find more information the 1.0 mg dose group.Metabolic Training For Weight Loss: Can Certain Exercises Shed Fat Much Faster? ➜
Lastly, in the post-tesofensine duration, rats got subcutaneous shots of saline. Given that the half-life of tesofensine has to do with 8 days, we continued reviewing the rats' performance for three even more days (S3 Fig, panel C). We observed no major adjustment in job efficiency, or the palatability actions sucrose generated throughout this period.- Quit eating when you really feel satisfied instead of waiting till you're annoyingly complete.
- There are materials in the body that can raise the price of injury repair work and also connective tissue repair service.
- Weight-loss medicines, like Semaglutide and Tirzepatide, supply appealing alternatives for people battling to slim down, yet optimizing their benefits needs a comprehensive technique.
- Semaglutide offers much more adaptability, being readily available in both injectable and dental forms.
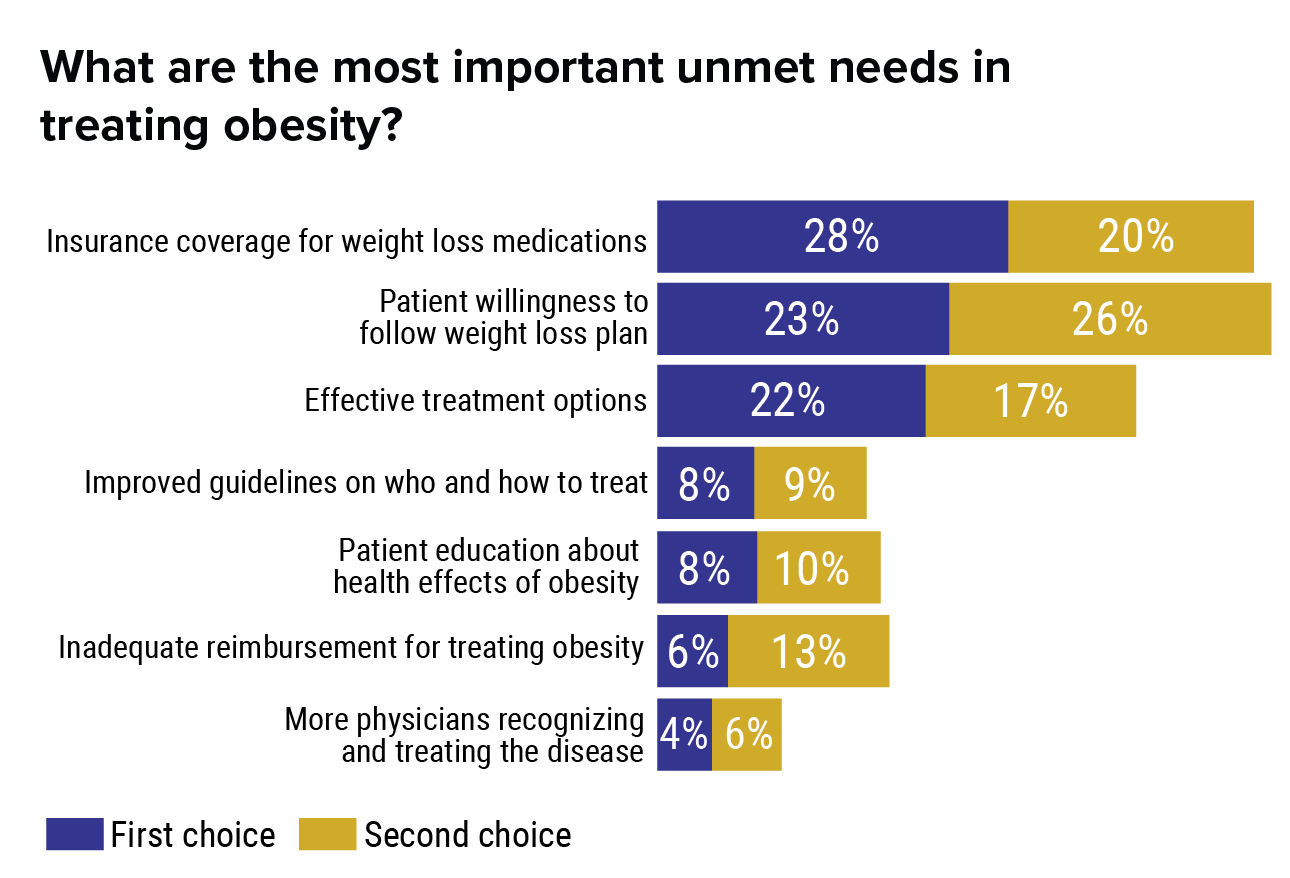
The Future Of Weight Reduction?
How many kilos can I lose with orlistat?
Social Links