
September 5, 2024
Component Three Next Generation Obesity Therapies
Healthcare Complimentary Full-text Medicinal Assistance For The Therapy Of Excessive Weight Present And Future To minimize damaging results of the doses called for to promote weight management, reduced dosage synergistic combinations such as GLP1R + glucagon or GIP are being investigated however have yet to be evaluated in huge confirmatory tests. Despite the indisputable metabolic benefits in rodent researches, FGF21 analogs have actually until now failed to meet assumptions in human beings. SGLT 1/2 inhibitors and AMPK/Sirt1 activators produce weight reduction with moderate unfavorable events however have yet to be examined in big tests of long period of time. The 10% weight management in 24 weeks generated by the centrally acting medicine Tesofensine is promising, however currently the product launch is expected just in Mexico and Argentina. The capacity for venous thromboembolism with MetAP2 inhibitors has resulted in a professional hang on its growth.Study Design And Individuals
What are the advanced obesity medicines?
Zepbound (tirzepatide), Wegovy (semaglutide), Saxenda (liraglutide), and much more are currently FDA accepted as fat burning therapies.
Glycerol-3-phosphate Acyltransferase Isoform-4 (gpat Restrictions Oxidation Of Exogenous Fats In Brown Adipocytes
As noted, our formula in control rats incorrectly misclassified grooming behavior as stereotypy in control rats. Nonetheless, no head weaving stereotypy was found under tesofensine 2 mg/kg, recommending, at the very least indirectly, a reduction in the possibility of brushing behavior. However, in uncommon circumstances, we observed that rats in a quiet-awake state would certainly also implement jaw and tongue activities, albeit at a lower strength (see S8 Video clip). Having actually revealed the neuronal correlates of tesofensine in the LH in rats and mice, we compared tesofensine appetite suppressant results with other cravings suppressants, particularly phentermine and 5-HTP. Ephedra has been utilized in Chinese medication for over 2,000 years and has 4isomers, the most powerful of which is ephedrine.- Having these three neurotransmitters prevented from being reabsorbed by the main nerve system causes the body sensation much less hungry.
- Practically a decade after excessive weight was categorized as a condition, leptin wasdiscovered and the concept of weight problems being a chronic, physiologically controlleddisease started to obtain traction [2]
- The pituitary gland depends on hypothalamic signals that are often disrupted from hypothalamic damage, that affects secretion of development hormone, gonadotropins, adrenocorticotrophic hormonal agent (ACTH) and thyroid stimulating hormone (TSH).
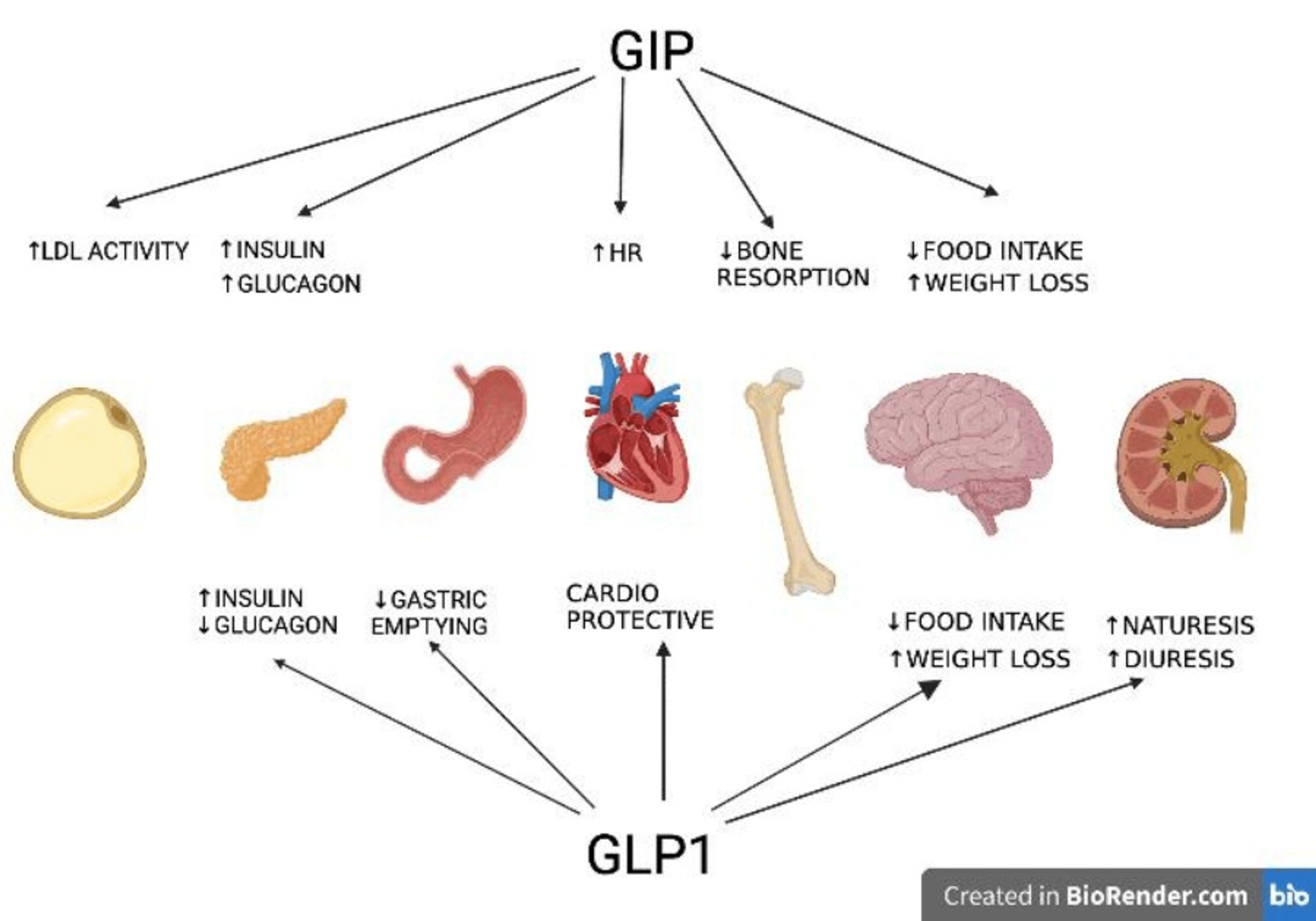
- . The mechanisms of action of glucagon-like peptide-1 agonists and co-agonists, diabetic issues medicines being checked out for weight loss, and medications acting upon the main nervous system in addition to peripherally are examined.
- Scientific studies and research study demonstrate the effectiveness of tesofensine in the domain name of weight reduction and weight problems monitoring.

Social Links