September 5, 2024
Randomized Regulated Trial Of Tesomet For Weight-loss In Hypothalamic Excessive Weight European Journal Of Endocrinology
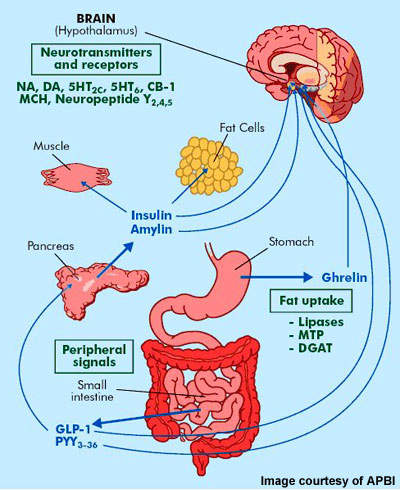
Box 1 Endocrine Control Of Food Intake
What is tesofensine used for?
- Right here, we define the results of tesofensine, an unique anti-obesity medicine that serves as a three-way monoamine natural chemical reuptake inhibitor.
- In a 54-week stage IIb research study in individuals with overweight and obesity with T2D, cotadutide reduced body weight and hepatic fat material and improved glucose tolerance relative to placebo198.
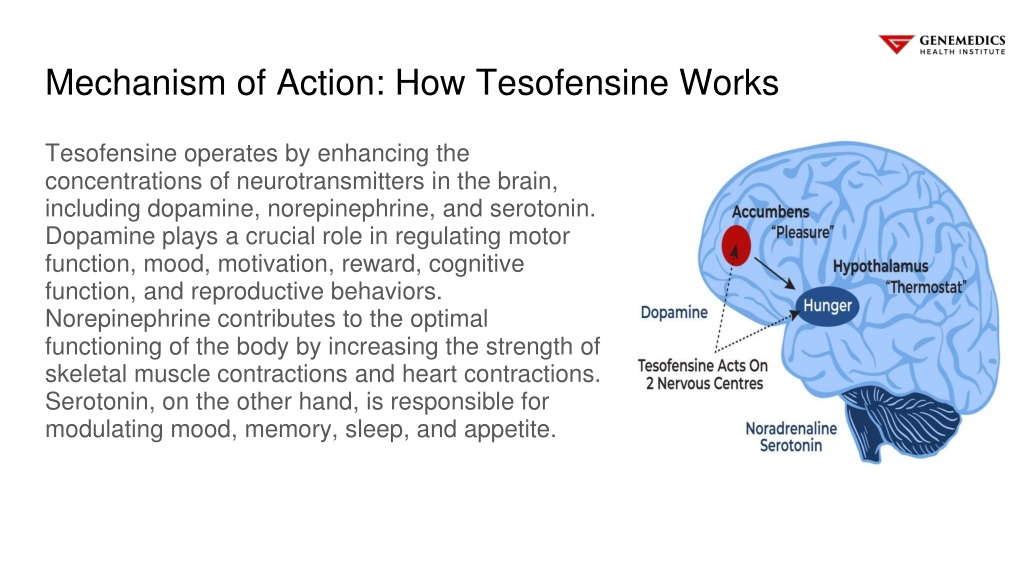
- Tesofensine functions largely as a hunger suppressant however might additionally increase resting power expense.
- The major modification observed during the tesofensine treatment was a shift in the distribution of tests finished on each quartile.
- People in the groups receiving tesofensine, 0.25 and 1 mg, skilled rises in on time with problematic dyskinesia.
The Anorexigenic Results Of Tesofensine Are Magnified By The Chemogenetic Restraint Of Lh Gabaergic Neurons
That these impacts are most likely to be dopaminergic is supported by positron discharge tomography showing blockade of the dopamine carrier leading to up-regulation of the dopamine path (Appel et al., 2014). It can be guessed that as elevated blood pressure was foreseeable from its mode of activity, this might have been managed with lower doses and an extra adaptable application program. In 2022, a stage 3 randomized, regulated medical trial demonstrated that tirzepatide led to a 20 percent reduction in body weight over 72 weeks. Food and Drug Administration to authorize the drug last month, with the brand name Zepbound, for weight reduction in individuals with a body mass index (BMI) of 30 or higher-- or for those with a BMI of 27 or higher that likewise had health problems such as high cholesterol or high blood pressure. Certainly, the medical outcomes with tirzepatide have caught wonderful focus and sustained rate of interest in GIP-based twin agonists and other combinatorial techniques.Emerging Therapies To Battle Obesity
Nevertheless, the precision of the sucrose discovery task (i.e., the percent right trials) was not considerably modified by tesofensine (S3 Fig). Additionally, it is well known that LH GABAergic stimulation normally results in stimulus-bound feeding. A lot of feeding happens within 2.5 secs of optogenetic excitement [11] (Fig 4D; Sal + laser). In an open loophole method (i.e., independently of actions), we located that tesofensine therapy reduced the number of licks however did not influence stimulus-bound feeding (Fig 4D, Teso + Laser), revealing that the drug per se did not hinder oromotor reflexes generated by optogenetic stimulation. These results demonstrate that the tesofensine-induced decrease in sucrose usage, measured by the variety of licks, is due to decreased feeding consummatory actions rather than merely hindering oromotor reflexes evoked by optogenetic excitement. T-distributed Stochastic Next-door neighbor Embedding (t-SNE) is an automatic dimensionality decrease approach that tries to group nerve cells with similar firing prices in a low-dimensional room to efficiently maintain neighborhood identity [36] But the only 2 currently readily available, Roche's Xenical (Orlistat) and Abbot's Reductil (Sibutramine), have adverse effects and are not especially efficient. It also creates damaged wheelchair, lowered warmth resistance, excessive sweating and skin folds up that can become infected. Yet it is linked in lethal illness varying from diabetes mellitus to kidney failure, heart failure and cardio problems. Whether you come to 4Ever Youthful Loudoun for Tesofensine therapy or any of our clinical weight management programs, you can expect substantial weight-loss and long-lasting results.Social Links